-
Posts
4,036 -
Joined
-
Last visited
-
Days Won
87
Posts posted by waterbear
-
-
I am moving your post to the Hot Tub Water Chemistry section of the forum.
How are you testing? Are you using DPD for testing bromine? (I want to eliminate a false high pH reading because of DPD bleachout) What is the pH and TA of your fill water. Main cause of pH rise is outgassing of CO2 had this is directly related to how low you place the pH and how high the TA is. With a TA of 50 you should have minimal outgassing but if you are lowering your pH below 7.6 this will cause pH to rise faster.
These posts will explain what is happening, read them:
https://www.poolspaforum.com/forum/index.php?/topic/52522-some-truths-about-ph-and-ta/
https://www.poolspaforum.com/forum/index.php?/topic/28846-lowering-total-alkalinity-howto/
-
Please post a full set of test results and how they were obtained (dealer testing, strips, liquid reagents, etc.)
10 hours ago, NanaJ said:PH and alkalinity are stable and in range.
What are the numbers? "In range" tells us nothing.
10 hours ago, NanaJ said:'m having to add shock every 1-2 days to keep CL and Br in range
Are you using chlorine or bromine? It's one or the other, not both. Even if you are adding chlorine to a bromine tub you will have bromine and not chlorine in the water.
Have you purged the spa? The facts that the water is cloudy and you are having trouble maintaining sanitizer level it usually indicated either biofilm or something 'growing' in the water.
Have you read the pinned posts at the top of the hot tub water chemistry section of the forum? Lots of good information there.
-
You would have found your answer in the pinned topics in this section of the forum. Start by reading these two the first one will explain the yo yo effect you are seeing and how to aviod it):
https://www.poolspaforum.com/forum/index.php?/topic/28846-lowering-total-alkalinity-howto/
https://www.poolspaforum.com/forum/index.php?/topic/52522-some-truths-about-ph-and-ta/
You might also find this post useful:
https://www.poolspaforum.com/forum/index.php?/topic/53410-how-to-use-bromine-3-step-method/
-
You posted in a 10 year old thread. You will have a better chance of getting an answer to you question if you start a new thread.
-
Don't worry, it's all good. You've gotten over a pretty steep learning curve very quickly. You now know that you can't test pH or TA when sanitizer is high and if your sanitizer reads low or nonexistent there is a chance your DPD test has bleached out and is reading low so dilute your sample with distilled water 1:1 and retest, multiply test results by 2 to get reading. (OR just get the Taylor stand alone FSD-DPD test kit. Much easier to use, no color matching. You are looking for it to go from pink to colorless as you add drops and count the drops. It will also handle a much higher sanitizer range than the DPD test and give you accurate results with NO dilutions.
Once the bromine drops don't put the floater back until we get the pH, TA, and CH in line. If you could post a pic of your bromine floater it would help, Some are totally useless as far as adjustments go. I recommend the Pentair 335 floater that holds 1" bromine tablets . It looks like this and is VERY adjustable. It's often sold unter the Rainbow brand, which is one of Pentair's brands.

-
You can't test pH or adjust it when sanitizer is over 10 ppm. Take out the floater and let the bromine drop (running the tub uncovered will help). In the mean time get a box of 20 mule team borax from the grocery story. Add 1/2 cup per 200 gallons. Wait for sanitizer to drop below 10 ppm, test pH and TA, and we'll take it from there. As for elemental bromine, it will fix itself once the pH is right. As far as the nitric acid, it will also fix itself once the pH rises but to keep it from reoccurring I would defer to @RDspaguy since his expertise is equipment repair. Let's see what he has to say.
-
@Samiko, you stand a better chance of getting your question answered if you start a new thread instead of posting in a thread that is over two years old.
-
On 9/6/2023 at 12:42 AM, RDspaguy said:
Nitric acid in the ozone tube from an undersized mazzei. Gets sucked in, coating everything in sticky yellow gunk and foaming like crazy.
Missed that the tub had ozone. You're probably right!
On 9/6/2023 at 12:42 AM, RDspaguy said:You know darn well I'm no chemist my friend. Elemental bromine? You give me too much credit.
You hold your own pretty well! Elemental bromine can form when the pH drops very low (which it looks like it did). It can color the water yellowish to brown and can stain, similar to Iodine. Has a sharp odor similar to chlorine and iodine. Toxic, similar to chlorine and iodine.
-
 1
1
-
-
4 hours ago, RDspaguy said:
Does it have a sharp, astringent odor?
I think we are on the same page, elemental bromine from very low pH.
-
13 hours ago, RDspaguy said:On 9/1/2023 at 4:02 PM, waterbear said:
TA will not rise on its own.
Aeration.
Aeration raises pH, not TA. Adding bicarbonate or carbonate raises TA, Acid lowers TA Aeration raises pH by gassng off CO2 so TA does not rise with the pH rise.
-
 1
1
-
-
Your attached videos do not play. I suspect they might be too large.
-
20 hours ago, TraceyM said:
it was showing real high PH. We added borax and it seemed to bring the numbers down
Impossible, borax will raise pH, not lower it. How much did you add? Are you still testing with the K-1005?
20 hours ago, TraceyM said:The following week we came back and the TA was super high as well as the PH. !added muratic acid to lower the TA (based on pool calculator)
TA will not rise on its own. It was probably the results of the borax you added previously. Borax has minimal impact on TA when compered to the sodium carbonate sold as pH increaser but it will sill cause a rise in the TA.
How much acid did you add? How many gallons is your spa? You CANNOT add all the acid at once since it will often lower the pH dangerously low and can cause damage to the pump, heater, and other parts and can cause elemental bromine to form in the water.
Read this to properly lower TA and to explain what happened when you added all the acid at once. It's the ONLY way that works!
https://www.poolspaforum.com/forum/index.php?/topic/28846-lowering-total-alkalinity-howto/
Read this for basic info on TA and pH.
https://www.poolspaforum.com/forum/index.php?/topic/52522-some-truths-about-ph-and-ta/
TA will change with pH and the pool calculator is not an accurate way to determine the amount of acid to lower pH since it can cause a very low pH conditions. Acid needs to be added in small increments with aeration between additions to lower TA. I suspect the very low pH you created cause elemental bromine to form in your tub. Get some sodium thiosulfate (photographer's hypo) from a photo store that sells darkroom chemicals or from an online retailer such as Amazon, Walmart (yes, they have it online only, The Chemistry Store, or a pool store (sold as a chlorine neutralizer). Get it in powdered form, either anhydrous or pentahydrate form, and dissolve 1 tablespoon in a pint of warm water and spray on the stain and let it sit for about a minute to see if it removes it. Rinse well and drain again before refilling or the thiosulfate will neutralize any sanitizer you add until it is depleted. The stain is similar to an iodine stain since they are in the same chemical family, along with chlorine and fluorine.
21 hours ago, TraceyM said:The first time I came back, it was showing real high PH
21 hours ago, TraceyM said:TA was super high as well as the PH
21 hours ago, TraceyM said:TA went a little lower but PH was over 8.0. We came back this week, opened the tub and the water is yellow.
It sounds like your floater might be open too much and is keeping your bromine levels too high . What was your bromine reading when you tested high pH? If it was low or nonexistent I suspect the DPD test in your K-1005 bleached out because of high bromine levels (which is why I prefer the FSD-DPD test in the K-2106 and k-2006 since it does not bleach out at high sanitizer levels up to about 40 ppm for bromine or 20 ppm for chlorine, you can buy a stand alone FAS-DPD test kit from Taylor Technologies, which will essentially convert your kit to a K-2006 (only the pH test would be different, the one in the K-2006 had better precision).
You can also dilute your sample before testing. Dilute 1 part spa water to 1 part distlled water (tap water can contain chlorine which will change your results), test the diluted sample and multiply the results by 2 to get your sanitizer reading. If it is at or above 20 ppm (bromine scale) the you pH test will not be accurate.
The reason I ask about sanitizer levels is high sanitizer levels (above about 10 ppm) WILL cause the pH test to read high since it will convert the phenol read indicator into bromphenol red which detects pH between 5.2 - 6.8 which means that if your comparator read that the pH was 8.0 or above all you really know is that the pH is 6.8
IF you have a way to measure 50 ml of water you can add 1 drop of reagent R-0007 (sodium thiosulfate) to the 50 ml sample, fill the pH comparator with the sample, and test again. If you still get the very high pH reading you will need to wait for the sanitizer to drop before testing pH.
21 hours ago, TraceyM said:What is it-algae? Biofilm? Am using the right stuff to clean it (Ahhsome spray?)
I suspect it is a stain from the elemental bromine that formed due to very low pH in your water. Keep in mine that I have not tested your water nor observed it or your tub or the stain so I am basing this on my knowlege of chemisty (not just poo/spa water chemistry).
Is the Ahhsome spray removing the staining? If not try the sodium thiosulfate, it's not expensive.
21 hours ago, TraceyM said:Once I get it cleaned, I will refill and try again. If not, anyone interested in a brand new hydropool, lol. I wish I knew how difficult this was going to be before spending all that $ on a headache that we have not been able to use since the drain! Change my mind….
Don't give up. I am here to help. If need be I will walk you through it again. It's really not that hard and you have been though some mistakes that you certainly won't repeat. Read my answers a few times since there is MUCH information in here explaining some of the testing "errors" you encountered. Most of the information I proved about testing came from the Taylor Technologies website regarding DPD testing and pH testing.
-
 1
1
-
-
@bgrvdub you are more likely to get an answer to your question if you start a new thread instead of tacking your question on a thread from 2010.
-
4 hours ago, Tinkers said:
Went with the 3 step bromine set up.
Are you using 3 step bromine or a salt water generator? I'm confused. If you are using 3 step bromine you can't be suing the mineral cartridge in your filter and your reading of .5 ppm FC is bogus.
4 hours ago, Tinkers said:Chlorine was showing 2 ppm and bromine 4-5 ppm.
If you are using bromine the chlorine is non existent since chlorine is converted to bromine by the bromine bank in the water. The same reagents measure chlorine, bromine and MPS but the scale is different. to convert a chlorine reading to bromine multiply by 2.25 so your reading of 2 ppm FC will be 4.5 ppm bromine,which is in line with what your posted for the bromine.
4 hours ago, Tinkers said:Were you meaning to say Bromine is a known SENSITIZER and chlorine is not?
Autocorrect strikes again! yes, I meant sensitizer
4 hours ago, Tinkers said:The tub is extremely clean. I have done numerous (6) Ahhsome purges and is just 1 year old. No gunk of any color or kind is coming out of the tub.
Clean looking water has NOTHING to do with whether there are pathogens growing in the water because there is not enough FAST ACTING residual sanitizer in the water (chlorine, bromine, biguinide, or silver/MPS) Copper is basically an algaecide with slow acting bacteriostatic prop[erties, as is zinc. The fallacy that they allow running a low FC level causes many problems. Ozone is a non residual oxidizer and there should be non in the actual tub since it is a sensitizer and lung irritant. Bottom line, ozone is toxic. The downside is that only a small amount of water in the reaction chamber interacts with the ozone so you still need a residual fast acting sanitizer/oxidizer in the water.
The average bather involuntarily releases 5–10 mL of urine in the pool or spa and 10–25 mg of fecal material every time they enter the water. (these numbers
courtesy of Taylor Technologies). Once again, this highlghts the need for a FAST ACTING residual sanitizer at a high enough level to deal with these. .5 ppm FC is quickly depeleted, leaving you no sanitizer in the water. Also, everyone sweats in the water (particualrly with a spa) and sweat and urine have a very similar chemical composition, depleting residual sanitizer even faster.
4 hours ago, Tinkers said:"poison ivy" looking rash
Did it look anything like this? This is a picture of hot tub Folliculitis (Hot tub itch) usually caused by pseudomonas or other pathogens growing in undersantized water.
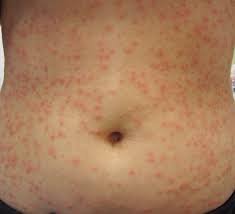
-
6 hours ago, Tinkers said:
If I decide to use dead sea salt in place of regular pool salt in my tub will I be creating a bromine tub or a chlorine tub since dead sea salt has bromides a
s well as other minerals in it?
Bromine. If you decide to try it make sure your salt water generator can use dead sea salts or run bromine. Not all can.
6 hours ago, Tinkers said:keep chlorine levels low as possible due to my wife's HIGH sensitivity to chlorine and bromine.
Do you MPS for shocking the tub? MPS is a KNOWN sensitizer and eliminating it from your tub maintenance can solve many sensitivity issues. Bromine is a known sanitizer, chlorine is not and ,in fact, bleach baths are often prescribed for babies and young children to treat eczema. The recommended dosing workes out to between 25 and 50 ppm. https://www.mayoclinic.org/diseases-conditions/atopic-dermatitis-eczema/expert-answers/eczema-bleach-bath/faq-20058413
6 hours ago, Tinkers said:she can't have boric acid in the tub or pool as well due to skin issues.
What type of skin issues? My concern is that you are depending on slow acting copper and zinc to sanitize the water and have a very low reserve of a quick acting sanitizer in the water.
6 hours ago, Tinkers said:The tub also has an ozonator.
Ozone depletes chlorine and if there is residual ozone in the water that is also a known sensitizer.
From what you have said you I would say that have an undersanitized tub that will allow the growth of pseudomonas and other pathogens in the water. Pseudomonas is the pathogen that causes hot tub itch.
Exactly what type of reaction does your wife have from chlorine and boric acid?
Also be aware that copper is what causes green hair in pools and spas, particularly if the hair is porous ,bleached, tinted, permed or straightened, or damaged.
-
Colored fiberglass fades from the sun. Period. End of Story. White fiberglass is always your best bet and will look blue from the refraction of sunlight by the water, as you can see from these pics of my white fiberglass pool.
2 hours ago, Lafayette_ Tim said:Thursday pools is pointing at saturation index
Calcium Saturation Index is a bogus measurement for fiberglass and vinyl pools. For a plaster or aggregate pool it's important since the pool surface is made of calcium and if there is too little in the water (pH also plays a factor) then it will be leached from the pool surface and etch it. If there is too much then you will have a problem with scale deposit and will need to acid wash. There is some anecdotal evidence that calcium too low could lead to colbalt spotting in fiberglass or leaching of plasticizer from vinyl but I have never seen anything conclusive. A CH of around 200 PPM seems to be all that is really needed.
3 hours ago, Lafayette_ Tim said:I believe it is sun damage fading the color.
Yep.
-
What is your current alkalinity and pH. Alkalinity will change with pH and aeration will cause pH to rise while keeping alkalinity constant.
-
-
@Sarah Fix you posted in a dead year old thread. Please start a new thread so you have a better chance of getting an answer. When you start your thread please post how you are sanitizing (chlorine tabs, salt water chlorine generator, liquid chlorine/bleach, diclor, cal hypo) and a full set of test results including cyanuric acid (stabilizer)m free chlorine, combined chlorine OR total chlorine , pH, total alkalinity and calcium hardness and if you use a non chlorine shock. Also post how the test results were obtained (test kit with liquid reagents, test strips, strips or reagents with a meter or strip reader, or dealer testing (please specify if they are using liquid reagents, a test disc in a meter, or strips (with or without a strip reader).
I have some ideas about what might be going on but need to know your water parameters first to narrow it down. I will answer in the new thread you start once you post the information.
Also, posting a picture of the 'slime' would be very helpful in identification.
That being said:
5 hours ago, Sarah Fix said:This has happened twice in 8 months and the only solution was acid washing the pool.
Which involved draining all the water, refilling and rebalancing the chemicals, which would temproarily correct any water balance problems such as overstabilization. I am going to guess that you are either using trichlor tabs in a feeder or floater or using dichlor for either chlorination or shocking. I am also going to guess that you have a cartridge filter or a non backwash DE filter. You are in Florida in St John's county so you do not close your pool. I am also in St Johns county so I am very aware of the special issues Florida pool owners face.
5 hours ago, Sarah Fix said:Our pool service indicates all chemical levels are normal
What is "normal"? What is their acceptable levels for cyanuric acid and free chlorine? A sad fact is that many pool service techs do not really understand the chemistry involved in pool maintenance and there are many pieces of misinformation that refuse to die in the the industry.
5 hours ago, Sarah Fix said:they have no idea what the cause is.
No surprise here. As I said many pool techs don't really understand pool water chemistry.
-
@vbslam, you have a better chance of getting an answer to your question if you start a new thread instead of tacking on to an old one.
-
 1
1
-
-
@marin_tubber, you posted in a 3 year old thread. You stand a better chance of getting your question answered if you start a new thread instead of tacking on to a dead thread.
-
When is the last time that you broke the filter down and cleaned the grids? They might be fouled. DE filtyers do need their grids cleaned periodically.
-
plain20 mule team borax will not cause foaming.
16 minutes ago, TraceyM said:I am using the Taylor 9 way Test Kit
Model # of the kit? Is it the K-1005 or K-2005? (K-1005 is ok but does not have the precision of the K-2005 for the pH and sanitizer tests, all other tests are identical). Since it has a comparator for sanitizer it is using DPD and not FAS-DPD (which is easier, more precise, does not bleach out at higher sanitizer levels, and wider range for testing. You can buy the Taylor FAS-DPD test as a stand alone kit, btw
26 minutes ago, TraceyM said:My PH today after aeration is now showing 7.8 but my TA is at 500
26 minutes ago, TraceyM said:My PH today after aeration is now showing 7.8 but my TA is at 500
I suspect it was testing error or a comparator tube that was not clean and rinsed out before one of your tests. retest 3 times and see if the resutls are the same (pH should be identical, TA should be within +/- 10 ppm with a 25 ml sample and +/- 25 ppm with a 10 ml sample.
-
how are you getting 3-6 on the bromine? are you using a comparator that has 32 on one side and 6 on the other? If so the 3 is a chlorine reading for those using chlorine and the 6 is your bromine reading. Both chlorine and bromine use the same test but the bromine reading is roughly twice the chlorine reading (actually 2.25 more).
pH is low. Does your test kit have a base demand test? If it does you can dose borax at twice the amount of sodium carbonate. However, with your TA at 100, I would start aerating the water (run the tub uncovered with all jets and aerators running and full air injection) and see if the pH starts to rise. I suspect it will unless you have added so much acid that your pH is WAY too low. Have you put acid in your tub?



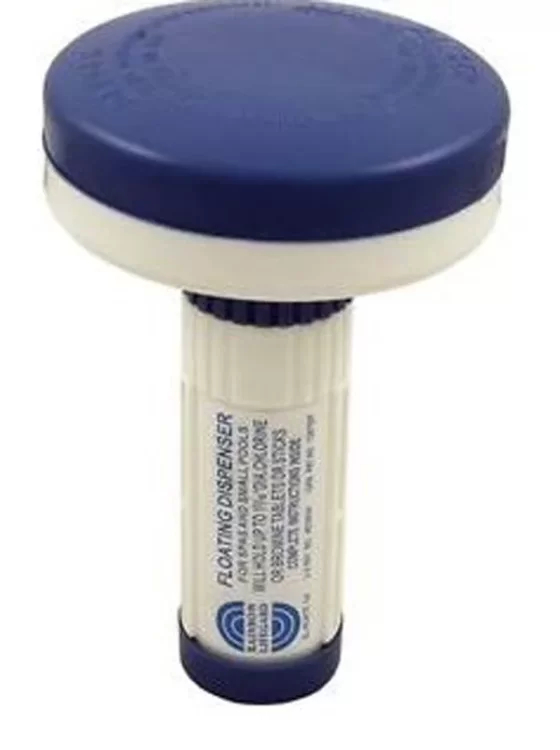


PH jumps above 8
in Hot Tub Water Chemistry
Posted
Not really. We are both well versed in chemistry so I don't want to go into too much depth here, but in a nutshell the carbonic acid/bicarbonate buffer will generally cause pH to rise as the carbonation is lost from outgassing. Henry's Law says that CO2 will off-gas until it reaches equilibrium with the air above the pool or spa. This the pH ceiling and it is around 8.2 for recreational water. If the TA is higher then there will be more carbonic acid at a given pH as the buffer reaches equilibrium and there is then more CO2 to outgas along with a faster pH rise as opposed to slower outgassing and slower pH rise at a lower TA since the overall carbonation of the water is lower.
FWIW, adding a secondary boric acid/borate buffer WILL slow the rise of pH which the main reason to add 50 ppm borate.